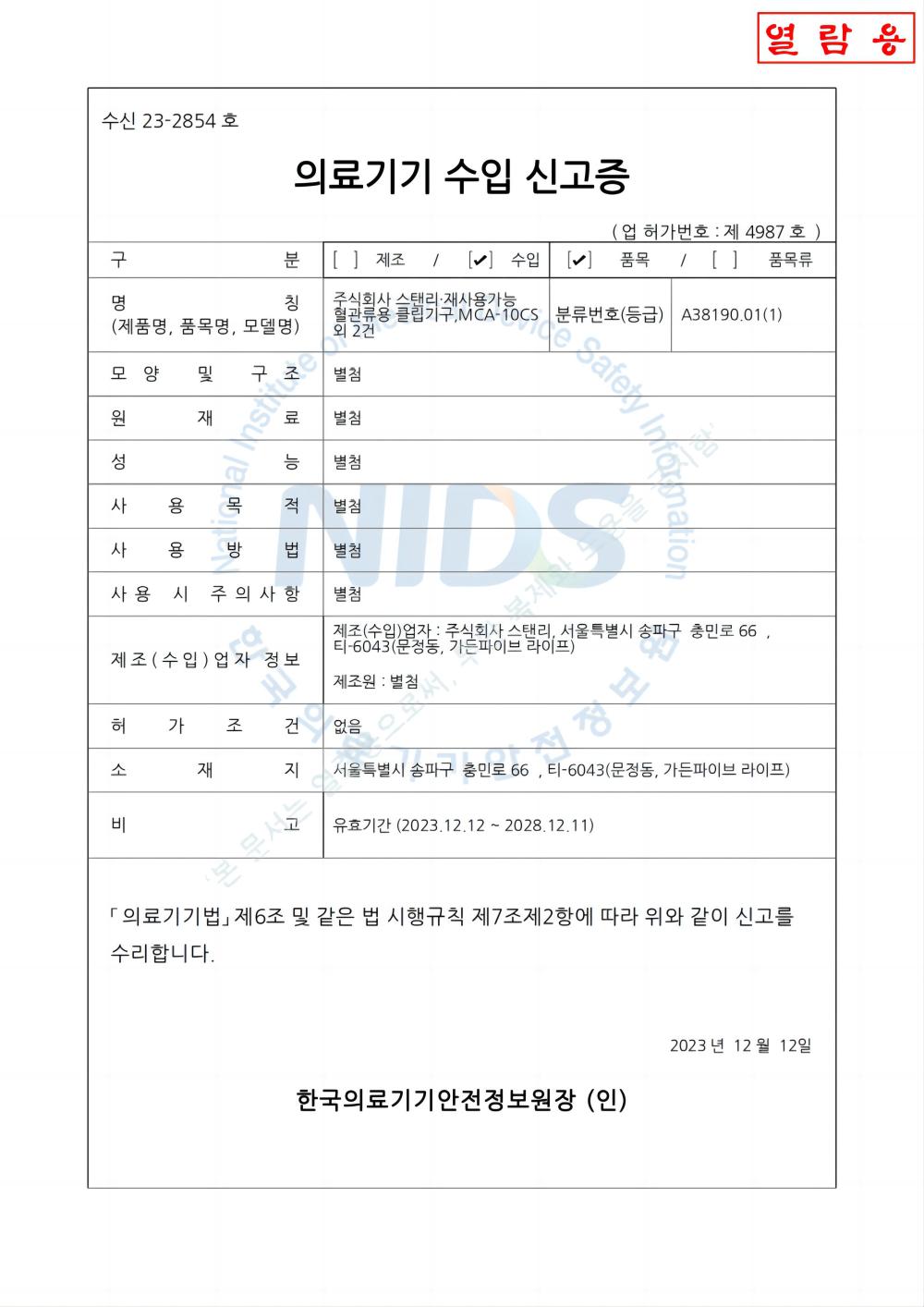
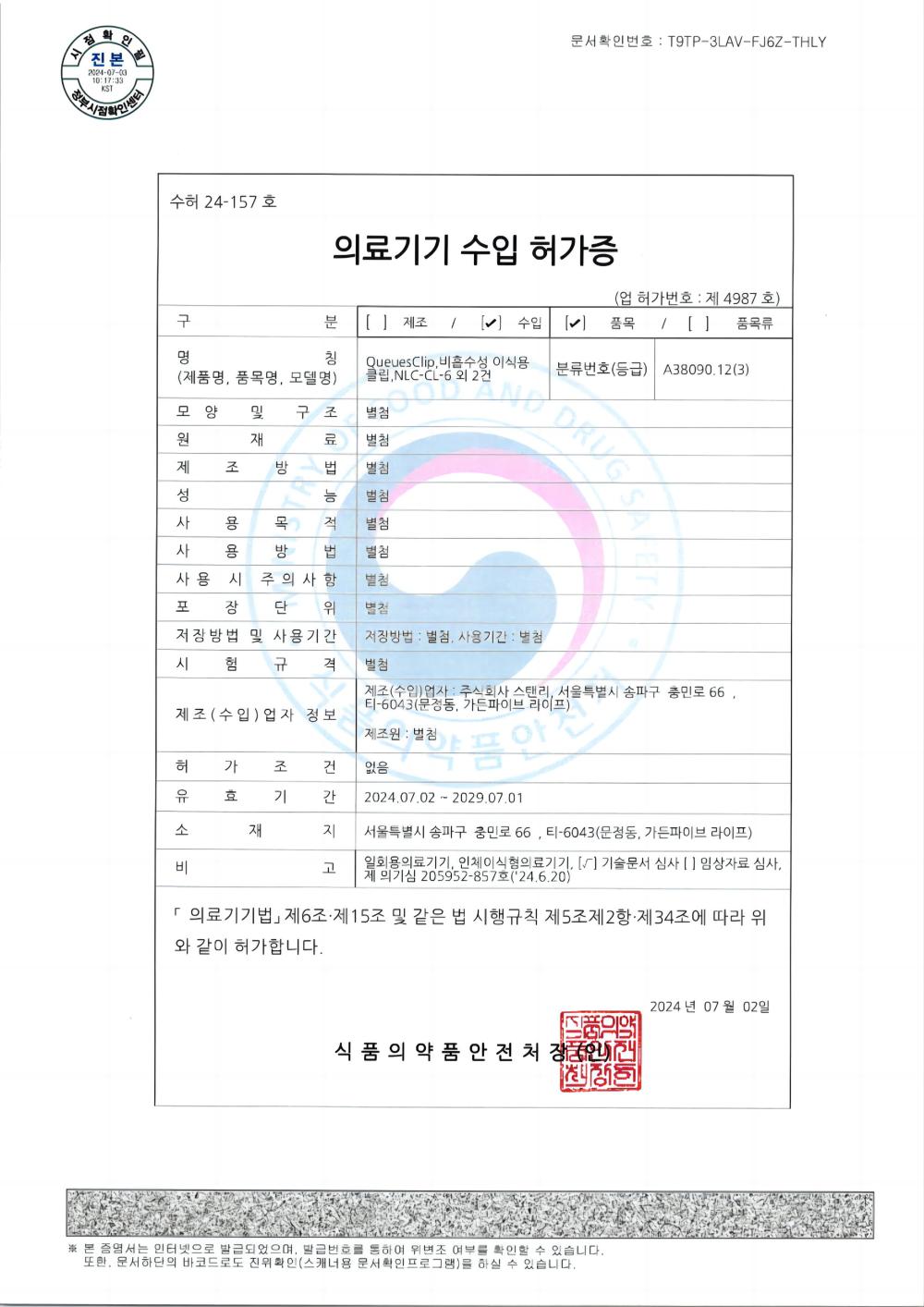
Tusipasi ole ISO13485
2024-09-04 11:43:33
O lo'o umia le tusi faamaonia Numera: MD 804964
ma fa'agaoioia se Faiga Fa'atonuga Lelei e fa'amalieina mana'oga o le ISO 13485:2016 mo le lautele o lo'o mulimuli mai:
Vaega Resitala:
O le Fuafuaga ma le Atina'e, Gaosi ma Fa'asoaina o mea fa'apipi'i fa'apipi'i fa'ato'a fa'ato'a fa'ato'a fa'ato'a fa'ato'a fa'ato'a fa'atosina le hemostatic lition clips, clip appliers, fa'apipi'i le tu'u fa'ameamea le tele o polymer Ligating kilipi, tele kilipa toe fa'aaogaina, fa'apipi'i tu'u fa'ameamea tu'ufua fa'ameamea tasi polymer Ligating clips ma le kilipi tasi toe fa'aaogaina mo taotoga lautele.
Le Fuafuaga ma le Atina'e, Gaosi ma Fa'asoaina o Sterile Biofragmentable Intestinal Staplers ma Intestine Gauges mo taotoga o le intestinal.
Uluai Aso Faamauina: 30-08-2024
Aso Fa'amamaluina: 30-08-2024
Aso Toe Iloiloga Fou: 30-08-2024
Aso Fa'amutaina: 29-08-2027
Igoa Kamupani ole Tagata talosaga/Laisene Nu.
Stanley Co., Ltd
Tulaga a le Kamupani a le Tagata talosaga
T-6043,66,Chungmin-ro, Songpa-gu,Seoul,Republic of Korea
Igoa o le Faumea
Hangzhou Sunstone Technology Co., Ltd.
Tuatusi o le Faumea
1st ma 2nd fogafale, fale 2,460 Fucheng Road, Qiantang New district, Hangzhou City, Zhejiang Province, Saina
Vaega
Meafaigaluega mo Faiga Faafomai
(Matou te faʻamaonia atu o loʻo tausisia e le kamupani gaosi oloa o loʻo taʻua i luga o Korea Good Manufacturing Practices of Medical Devices mo le vaega o oloa o loʻo lisiina i luga)
Aso o Fa'amatalaga: 2023.09.27
Aso e muta ai: 2026.09.26